Page 316 - Syndipharma
P. 316
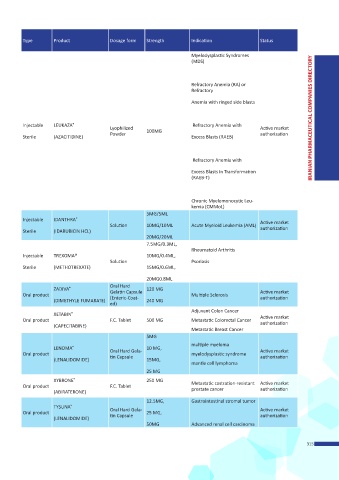
Type Product Dosage form Strength Indication Status
Myelodysplastic Syndromes
(MDS)
Refractory Anemia (RA) or
Refractory
Anemia with ringed side blasts
Injectable LEUKAZA ® Refractory Anemia with IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
Lyophilized Active market
Powder 100MG authorization
Sterile (AZACITIDINE) Excess Blasts (RAEB)
Refractory Anemia with
Excess Blasts in Transformation
(RAEB-T)
Chronic Myelomonocytic Leu-
kemia (CMMoL)
5MG/5ML
®
Injectable IDANTHRA Active market
Solution 10MG/10ML Acute Myeloid Leukemia (AML)
Sterile (IDARUBICIN HCL) authorization
20MG/20ML
7.5MG/0.3ML,
Rheumatoid Arthritis
Injectable TREXOMA® 10MG/0.4ML,
Solution Psoriasis
Sterile (METHOTREXATE) 15MG/0.6ML,
20MG0.8ML
Oral Hard
ZADIVA ® 120 MG
Oral product Gelatin Capsule Multiple Sclerosis Active market
authorization
(Enteric-Coat-
(DIMETHYLE FUMARATE) 240 MG
ed)
Adjuvant Colon Cancer
XETABIN ®
Oral product F.C. Tablet 500 MG Metastatic Colorectal Cancer Active market
authorization
(CAPECITABINE)
Metastatic Breast Cancer
5MG
multiple myeloma
LENOMA ® 10 MG,
Oral product Oral Hard Gela- myelodysplastic syndrome Active market
authorization
tin Capsule
(LENALIDOMIDE) 15MG,
mantle cell lymphoma
25 MG
XYBRONE ® 250 MG
Oral product F.C. Tablet Metastatic castration-resistant Active market
prostate cancer
authorization
(ABIRATERONE)
12.5MG, Gastrointestinal stromal tumor
TYSUNA ®
Oral product Oral Hard Gela- 25 MG, Active market
tin Capsule
authorization
(LENALIDOMIDE)
50MG Advanced renal cell carcinoma
315