Page 315 - Syndipharma
P. 315
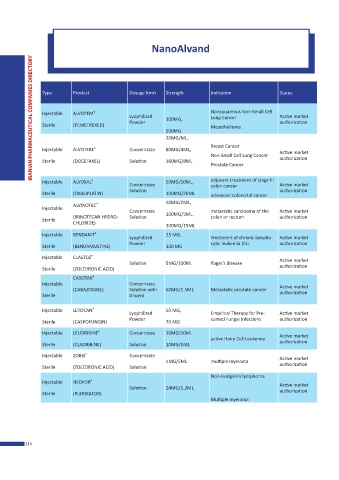
NanoAlvand
IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
Type Product Dosage form Strength Indication Status
Injectable ALVOPEM ® Lyophilized Nonsquamous Non-Small Cell Active market
Lung Cancer
Powder 100MG, authorization
Sterile (PEMETREXED) Mesothelioma
500MG
20MG/ML,
Breast Cancer
Injectable ALVOTERE ® Concentrate 80MG/4ML, Active market
Non-Small Cell Lung Cancer
Sterile (DOCETAXEL) Solution 160MG/8ML authorization
Prostate Cancer
Injectable ALVOXAL ® Concentrate 50MG/10ML, adjuvant treatment of stage III Active market
colon cancer
Solution authorization
Sterile (OXALIPLATIN) 100MG/20ML advanced colorectal cancer
40MG/2ML,
ALVINOTEC ®
Injectable
Concentrate metastatic carcinoma of the Active market
(IRINOTECAN HYDRO- Solution 100MG/5ML, colon or rectum authorization
Sterile
CHLORIDE)
300MG/15ML
Injectable BENDANIT ® 25 MG,
Lyophilized Treatment of chronic lympho- Active market
Sterile (BENDAMUSTINE) Powder 100 MG cytic leukemia (CLL authorization
Injectable CLASTOZ ® Active market
Solution 5MG/100ML Paget’s disease
Sterile (ZOLEDRONIC ACID) authorization
CABOTAX ®
Injectable Concentrate Active market
(CABAZITAXEL) Solution with 60MG/1.5ML Metastatic prostate cancer
Sterile Diluent authorization
Injectable LETOCAN ® 50 MG,
Lyophilized Empirical Therapy for Pre- Active market
Powder sumed Fungal Infections authorization
Sterile (CASPOFUNGIN) 70 MG
Injectable LEUDRIBINE Concentrate 10MG/10ML Active market
®
active Hairy Cell Leukemia
Sterile (CLADRIBINE) Solution 10MG/5ML authorization
Injectable ZOBIS ® Concentrate Active market
4MG/5ML multiple myeloma
Sterile (ZOLEDRONIC ACID) Solution authorization
Non-Hodgkin’s lymphoma
Injectable NEOFOR ® Active market
Solution 24MG/1.2ML
Sterile (PLERIXAFOR) authorization
Multiple myeloma
314