Page 204 - book
P. 204
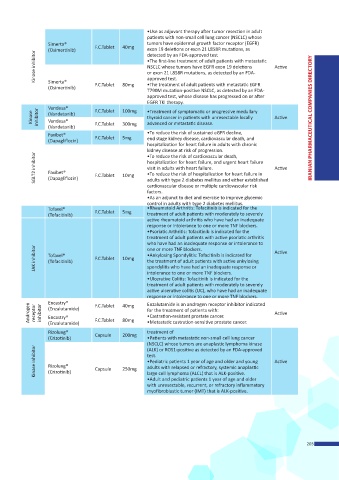
•Use as adjuvant therapy after tumor resection in adult
patients with non-small cell lung cancer (NSCLC) whose
Simerta® F.C.Tablet 40mg tumors have epidermal growth factor receptor (EGFR)
(Osimertinib) exon 19 deletions or exon 21 L858R mutations, as
Kinase inhibitor •The first-line treatment of adult patients with metastatic Active
detected by an FDA-approved test
NSCLC whose tumors have EGFR exon 19 deletions
or exon 21 L858R mutations, as detected by an FDA-
Simerta®
•The treatment of adult patients with metastatic EGFR
(Osimertinib) F.C.Tablet 80mg approved test.
T790M mutation-positive NSCLC, as detected by an FDA-
approved test, whose disease has progressed on or after
EGFR TKI therapy.
Vandesa® F.C.Tablet 100mg •Treatment of symptomatic or progressive medullary
Kinase inhibitor (Vandetanib) F.C.Tablet 300mg thyroid cancer in patients with unresectable locally Active IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
Vandesa®
advanced or metastatic disease.
(Vandetanib)
Faxibet® F.C.Tablet 5mg •To reduce the risk of sustained eGFR decline,
(Dapagliflozin) end stage kidney disease, cardiovascular death, and
hospitalization for heart failure in adults with chronic
kidney disease at risk of progression.
SGLT2 inhibitor Faxibet® F.C.Tablet 10mg hospitalization for heart failure, and urgent heart failure Active
•To reduce the risk of cardiovascular death,
visit in adults with heart failure.
•To reduce the risk of hospitalization for heart failure in
(Dapagliflozin)
adults with type 2 diabetes mellitus and either established
cardiovascular disease or multiple cardiovascular risk
factors.
•As an adjunct to diet and exercise to improve glycemic
control in adults with type 2 diabetes mellitus.
Tofaxel® F.C.Tablet 5mg •Rheumatoid Arthritis: Tofacitinib is indicated for the
(Tofacitinib) treatment of adult patients with moderately to severely
active rheumatoid arthritis who have had an inadequate
response or intolerance to one or more TNF blockers.
•Psoriatic Arthritis: Tofacitinib is indicated for the
treatment of adult patients with active psoriatic arthritis
who have had an inadequate response or intolerance to
JAK inhibitor Tofaxel® F.C.Tablet 10mg •Ankylosing Spondylitis: Tofacitinib is indicated for Active
one or more TNF blockers.
the treatment of adult patients with active ankylosing
(Tofacitinib)
spondylitis who have had an inadequate response or
intolerance to one or more TNF blockers.
•Ulcerative Colitis: Tofacitinib is indicated for the
treatment of adult patients with moderately to severely
active ulcerative colitis (UC), who have had an inadequate
response or intolerance to one or more TNF blockers.
Encastry®
Enzalutamide is an androgen receptor inhibitor indicated
Androgen receptor inhibitor (Enzalutamide) F.C.Tablet 40mg for the treatment of patients with: Active
•Castration-resistant prostate cancer.
Encastry®
F.C.Tablet
80mg
•Metastatic castration-sensitive prostate cancer.
(Enzalutamide)
Rizolung® Capsule 200mg treatment of
(Crizotinib) •Patients with metastatic non-small cell lung cancer
(NSCLC) whose tumors are anaplastic lymphoma kinase
Kinase inhibitor Rizolung® Capsule 250mg test. Active
(ALK) or ROS1-positive as detected by an FDA-approved
•Pediatric patients 1 year of age and older and young
adults with relapsed or refractory, systemic anaplastic
(Crizotinib)
large cell lymphoma (ALCL) that is ALK-positive.
•Adult and pediatric patients 1 year of age and older
with unresectable, recurrent, or refractory inflammatory
myofibroblastic tumor (IMT) that is ALK-positive.
203