Page 203 - book
P. 203
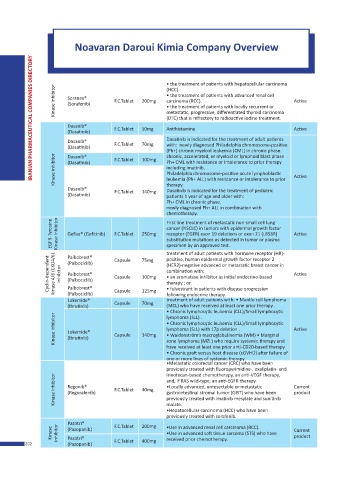
Noavaran Daroui Kimia Company Overview
IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
• the treatment of patients with hepatocellular carcinoma
Kinase inhibitor Soranex® F.C.Tablet 200mg • the treatment of patients with advanced renal cell Active
(HCC).
carcinoma (RCC).
(Sorafenib)
• the treatment of patients with locally recurrent or
metastatic, progressive, differentiated thyroid carcinoma
(DTC) that is refractory to radioactive iodine treatment.
Dasanib® F.C.Tablet 50mg Antihistamine Active
(Dasatinib)
Dasanib® F.C.Tablet 70mg Dasatinib is indicated for the treatment of adult patients
(Dasatinib) with: newly diagnosed Philadelphia chromosome-positive
(Ph+) chronic myeloid leukemia (CML) in chronic phase.
chronic, accelerated, or myeloid or lymphoid blast phase
Dasanib®
Kinase inhibitor (Dasatinib) Ph+ CML with resistance or intolerance to prior therapy Active
F.C.Tablet
100mg
including imatinib.
Philadelphia chromosome-positive acute lymphoblastic
leukemia (Ph+ ALL) with resistance or intolerance to prior
Dasanib® F.C.Tablet 140mg therapy.
Dasatinib is indicated for the treatment of pediatric
(Dasatinib) patients 1 year of age and older with:
Ph+ CML in chronic phase.
newly diagnosed Ph+ ALL in combination with
chemotherapy.
EGFR-Tyrosine Kinase inhibitor Gefisa® (Gefitinib) F.C.Tablet 250mg cancer (NSCLC) in tumors with epidermal growth factor Active
First-line treatment of metastatic non-small cell lung
receptor (EGFR) exon 19 deletions or exon 21 (L858R)
substitution mutations as detected in tumor or plasma
specimen by an approved test.
treatment of adult patients with hormone receptor (HR)-
Cyclin-dependent kinase 4/6 (CKD4/6) inhibitor Palbobrest® Capsule 75mg positive, human epidermal growth factor receptor 2 Active
(Palbociclib)
(HER2)-negative advanced or metastatic breast cancer in
combination with:
Palbobrest®
100mg
• an aromatase inhibitor as initial endocrine-based
Capsule
(Palbociclib)
therapy ; or
Palbobrest®
(Palbociclib)
following endocrine therapy.
treatment of adult patients with: • Mantle cell lymphoma
Lokemide® Capsule 125mg • fulvestrant in patients with disease progression
70mg
Capsule
(Ibrutinib) (MCL) who have received at least one prior therapy .
• Chronic lymphocytic leukemia (CLL)/Small lymphocytic
Kinase inhibitor Lokemide® Capsule 140mg • Chronic lymphocytic leukemia (CLL)/Small lymphocytic Active
lymphoma (SLL) .
lymphoma (SLL) with 17p deletion .
• Waldenström’s macroglobulinemia (WM) • Marginal
(Ibrutinib)
zone lymphoma (MZL) who require systemic therapy and
have received at least one prior anti-CD20-based therapy
• Chronic graft versus host disease (cGVHD) after failure of
one or more lines of systemic therapy
•Metastatic colorectal cancer (CRC) who have been
previously treated with fluoropyrimidine-, oxaliplatin- and
irinotecan-based chemotherapy, an anti-VEGF therapy,
Kinase inhibitor Regonib® F.C.Tablet 40mg and, if RAS wild-type, an anti-EGFR therapy. Current
•Locally advanced, unresectable or metastatic
gastrointestinal stromal tumor (GIST) who have been
product
(Regorafenib)
previously treated with imatinib mesylate and sunitinib
malate.
•Hepatocellular carcinoma (HCC) who have been
previously treated with sorafenib.
Pazotri® F.C.Tablet 200mg •Use in advanced renal cell carcinoma (RCC).
Kinase inhibitor (Pazopanib) •Use in advanced soft tissue sarcoma (STS) who have Current
product
Pazotri®
202 (Pazopanib) F.C.Tablet 400mg received prior chemotherapy.