Page 290 - Syndipharma
P. 290
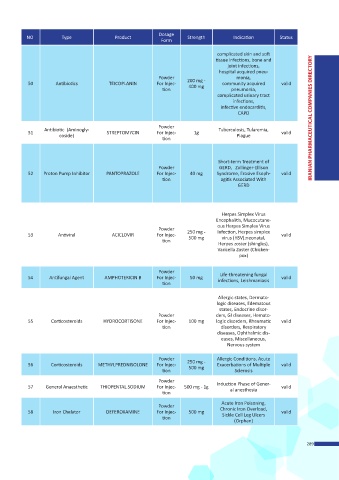
Dosage
NO. Type Product Strength Indication Status
Form
complicated skin and soft
tissue infections, bone and
joint infections,
hospital acquired pneu-
Powder monia,
50 Antibiotics TEICOPLANIN For Injec- 200 mg - community acquired valid
400 mg
tion pneumonia,
complicated urinary tract
infections,
infective endocarditis,
CAPD IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
Powder
Antibiotic (Aminogly- Tuberculosis, Tularemia,
51 STREPTOMYCIN For Injec- 1g valid
coside) Plague
tion
Short-term Treatment of
Powder GERD, Zollinger-Ellison
52 Proton Pump Inhibitor PANTOPRAZOLE For Injec- 40 mg Syndrome, Erosive Esoph- valid
tion agitis Associated With
GERD
Herpes Simplex Virus
Encephalitis, Mucocutane-
ous Herpes Simplex Virus
Powder 250 mg - Infection, Herpes simplex
53 Antiviral ACICLOVIR For Injec- 500 mg virus (HSV);neonatal, valid
tion
Herpes zoster (shingles),
Varicella Zoster (Chicken-
pox)
Powder
54 Antifungal Agent AMPHOTERICIN B For Injec- 50 mg Life-threatening fungal valid
tion infections, Leishmaniasis
Allergic states, Dermato-
logic diseases, Edematous
states, Endocrine disor-
Powder ders, GI diseases, Hemato-
55 Corticosteroids HYDROCORTISONE For Injec- 100 mg logic disorders, Rheumatic valid
tion disorders, Respiratory
diseases, Ophthalmic dis-
eases, Miscellaneous,
Nervous system
Powder Allergic Conditions, Acute
56 Corticosteroids METHYLPREDNISOLONE For Injec- 250 mg - Exacerbations of Multiple valid
500 mg
tion Sclerosis
Powder
57 General Anaesthetic THIOPENTAL SODIUM For Injec- 500 mg - 1g Induction Phase of Gener- valid
al anesthesia
tion
Acute Iron Poisoning,
Powder
58 Iron Chelator DEFEROXAMINE For Injec- 500 mg Chronic Iron Overload, valid
Sickle Cell Leg Ulcers
tion
(Orphan)
289