Page 265 - Syndipharma
P. 265
Faran Shimi Co.
IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
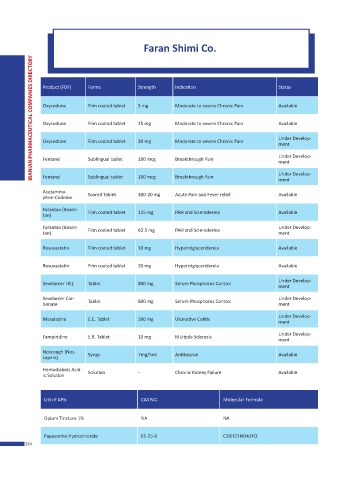
Product (FDF) Forms Strength Indication Status
Oxycodone Film coated tablet 5 mg Moderate to severe Chronic Pain Available
Oxycodone Film coated tablet 15 mg Moderate to severe Chronic Pain Available
Under Develop-
Oxycodone Film coated tablet 30 mg Moderate to severe Chronic Pain
ment
Fentanyl Sublingual tablet 100 mcg Breakthrough Pain Under Develop-
ment
Under Develop-
Fentanyl Sublingual tablet 100 mcg Breakthrough Pain
ment
Acetamino- Scored Tablet 300-20 mg Acute Pain and Fever relief Available
phen-Codeine
Farantan (Bosen- Film coated tablet 125 mg PAH and Scleroderma Available
tan)
Farantan (Bosen- Film coated tablet 62.5 mg PAH and Scleroderma Under Develop-
tan) ment
Rosuvastatin Film coated tablet 10 mg Hypertriglyceridemia Available
Rosuvastatin Film coated tablet 20 mg Hypertriglyceridemia Available
Under Develop-
Sevelamer HCL Tablet 800 mg Serum Phosphorus Control
ment
Sevelamer Car- Tablet 800 mg Serum Phosphorus Control Under Develop-
bonate ment
Under Develop-
Mesalazine E.C. Tablet 500 mg Ulcerative Colitis
ment
Under Develop-
Fampiridine E.R. Tablet 10 mg Multiple Sclerosis
ment
Noscough (Nos- Syrup 7mg/5ml Antitussive Available
capine)
Hemodialysis Acid- Solution - Chronic Kidney Failure Available
ic Solution
List of APIs CAS NO. Molecular Formula
Opium Tincture 1% NA NA
Papaverine Hydrochloride 61-25-6 C20H21NO4.HCL
264