Page 243 - book
P. 243
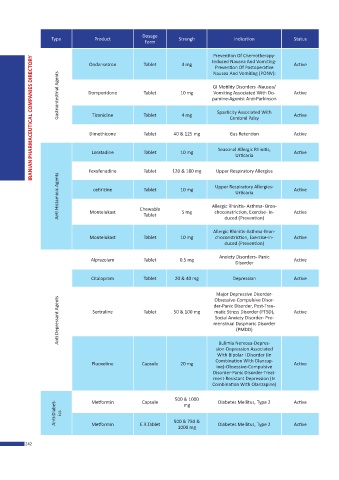
Dosage
Type Product Strengh Indication Status
Form
Prevention Of Chemotherapy-
IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
Induced Nausea And Vomiting-
Ondansetron Tablet 4 mg Active
Prevention Of Postoperative
Nausea And Vomiting (PONV):
Gastrointestinal Agents Domperidone Tablet 10 mg pamine-Agonist Anti-Parkinson Active
GI Motility Disorders -Nausea/
Vomiting Associated With Do-
Spasticity Associated With
Tizanidine
4 mg
Tablet
Dimethicone Tablet 40 & 125 mg Cerebral Palsy Active
Gas Retention
Active
Seasonal Allergic Rhinitis,
Loratadine Tablet 10 mg Active
Urticaria
Fexofenadine Tablet 120 & 180 mg Upper Respiratory Allergies
Anti Histaminic Agents Montelukast Chewable 10 mg Allergic Rhinitis- Asthma- Bron- Active
Upper Respiratory Allergies-
cetirizine
Tablet
Urticaria
5 mg
choconstriction, Exercise- In-
Active
Tablet
duced (Prevention)
Allergic Rhinitis-Asthma-Bron-
Montelukast Tablet 10 mg choconstriction, Exercise-In- Active
duced (Prevention)
Anxiety Disorders- Panic
Alprazolam Tablet 0.5 mg Active
Disorder
Citalopram Tablet 20 & 40 mg Depression Active
Major Depressive Disorder-
Anti Depressant Agents Sertraline Tablet 50 & 100 mg menstrual Dysphoric Disorder Active
Obsessive-Compulsive Disor-
der-Panic Disorder, Post-Trau-
matic Stress Disorder (PTSD),
Social Anxiety Disorder- Pre-
(PMDD)
Bulimia Nervosa-Depres-
sion-Depression Associated
With Bipolar I Disorder (In
Combination With Olanzap-
Fluoxetine Capsule 20 mg ine)-Obsessive-Compulsive Active
Disorder-Panic Disorder-Treat-
ment-Resistant Depression (In
Combination With Olanzapine)
500 & 1000 Diabetes Mellitus, Type 2 Active
Capsule
Metformin
Anti-Diabet- ics
mg
500 & 750 &
E.R.Tablet
Metformin
1000 mg Diabetes Mellitus, Type 2 Active
242