Page 126 - book
P. 126
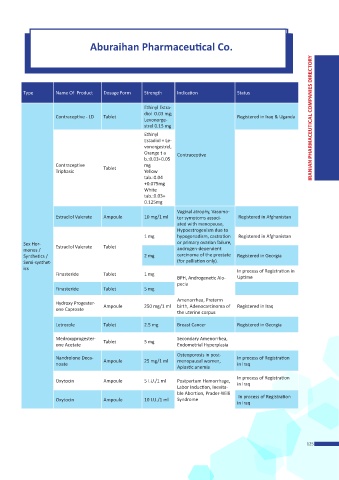
Aburaihan Pharmaceutical Co.
Type Name Of Product Dosage Form Strength Indication Status
Ethinyl Estra-
diol 0.03 mg;
Contraceptive - LD Tablet Registered in Iraq & Uganda
Levonorge- IRANIAN PHARMACEUTICAL COMPANIES DIRECTORY
strel 0.15 mg
Ethinyl
Estadiol + Le-
vonorgestrel,
Orange t a Contraceptive
b.:0.03+0.05
Contraceptive mg
Triphasic Tablet Yellow
tab.:0.04
+0.075mg
White
tab.:0.03+
0.125mg
Vaginal atrophy, Vasomo-
Estradiol Valerate Ampoule 10 mg/1 ml tor symptoms associ- Registered in Afghanistan
ated with menopause,
Hypoestrogenism due to
1 mg hypogonadism, castration Registered in Afghanistan
Sex Hor- Estradiol Valerate Tablet or primary ovarian failure,
mones / androgen-dependent
Synthetics / 2 mg carcinoma of the prostate Registered in Georgia
Semi-synthet- (for palliation only).
ics In process of Registration in
Finasteride Tablet 1 mg
BPH, Androgenetic Alo- Uptime
pecia
Finasteride Tablet 5 mg
Amenorrhea, Preterm
Hydroxy Progester-
one Caproate Ampoule 250 mg/1 ml birth, Adenocarcinoma of Registered in Iraq
the uterine corpus
Letrozole Tablet 2.5 mg Breast Cancer Registered in Georgia
Medroxyprogester- Secondary Amenorrhea,
one Acetate Tablet 5 mg Endometrial Hyperplasia
Osteoporosis in post-
Nandrolone Deca- In process of Registration
noate Ampoule 25 mg/1 ml menopausal women, in Iraq
Aplastic anemia
In process of Registration
Oxytocin Ampoule 5 I.U./1 ml Postpartum Hemorrhage, in Iraq
Labor Induction, Inevita-
ble Abortion, Prader-Willi
Oxytocin Ampoule 10 I.U./1 ml Syndrome In process of Registration
in Iraq
125